COVID drug Molnupiravir expected to hit Chinese market this week

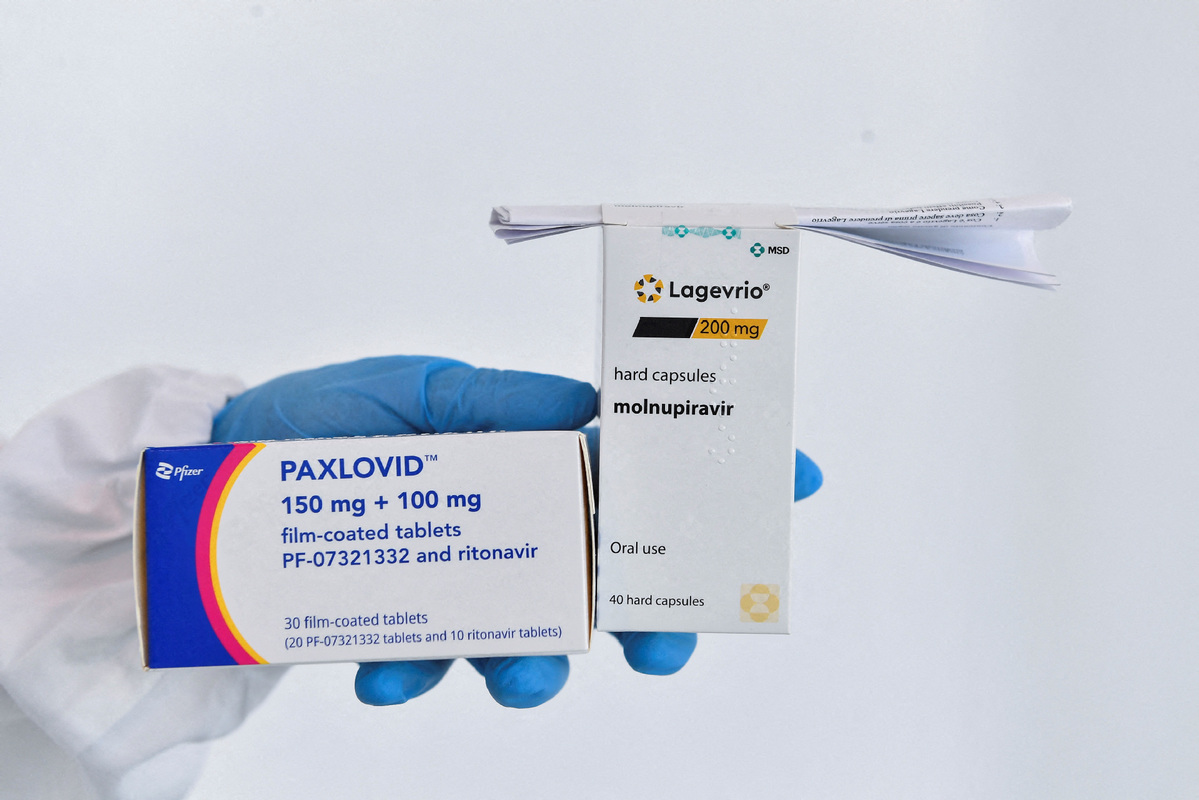
Molnupiravir, a COVID-19 drug developed by US pharmaceutical company Merck is expected to go into market in China on Friday, according to Liu Yong, president of Sinopharm Group.
Liu said Molnupiravir has passed the test for first-time imported drugs on Wednesday and Sinopharm will be responsible for selling the drug in the country, Knews, the news portal of Shanghai Media Group first reported on Wednesday.
The company confirmed with China Daily on Wednesday of the authenticity of the report.
Liu, who is a deputy to Shanghai Municipal People's Congress, made the remarks on Wednesday during the ongoing session of the congress.
The National Medical Products Administration has granted conditional market approval for the import of Molnupiravir on Dec 29, according to a release from the administration published on Dec 30.
The drug can be used for an adult COVID-19 patient who has mild to moderate symptoms and has high risks to develop into severe patients, such as old age, obesity, chronic kidney disease and diabetes, the release said.
- Report on Shenzhen-Jiangmen railway collapse documents regulation violations
- GX Foundation opens its global headquarters in Hong Kong
- China's success in development empowers Global South
- Study explains why Chang'e 6 moon soil is unexpectedly sticky
- Rare Sapria himalayana once again enters blooming period in Yunnan
- Shenzhou XXII to launch with full cargo load